Queensland and Oxford: a tale of two vaccine agreements

Biotech company CSL Limited has signed two separate heads of agreement for the supply of COVID-19 vaccines: one with the Australian Government for the supply of 51 million doses of the University of Queensland’s (UQ) COVID-19 vaccine candidate (V451) and another with AstraZeneca to manufacture the Oxford University candidate (AZD1222). Since the signing of the agreements, the ABC has reported that the Oxford vaccine trial is on hold following a potentially unexplained illness in one of the trial participants. AstraZeneca will conduct an independent review of the illness before continuing with the trial.
The UQ vaccine agreement
The number of vaccines ordered is based on a two dose per person regime. Upon completion of successful clinical trials, CSL expects the first batch of doses to be available by mid-2021.
CSL CEO and Managing Director Paul Perreault said, “The social and economic impact of the COVID-19 pandemic has brought a high level of urgency to the task of developing a vaccine against the SARS-CoV-2 virus and to manufacture a successful vaccine at high quality and in sufficient quantities.
“CSL has been working at pace to respond to the pandemic and has invested significant resources in the rapid development and large-scale manufacture of UQ-CSL V451, along with a number of other therapeutic programs.
“Together with partners including the University of Queensland and Coalition for Epidemic Preparedness (CEPI), our development and manufacturing teams have been working extremely hard to advance this program to ensure the availability of a safe and effective vaccine should clinical studies prove successful,” he said.
The heads of agreement is between CSL’s influenza vaccines company Seqirus — which will hold regulatory responsibility as the marketing authorisation holder — and the Australian Government. Production of the vaccine to support late-stage clinical trials has commenced at CSL’s biotech manufacturing facilities in Broadmeadows, Melbourne.
Results from the pre-clinical and early clinical studies for UQ-CSL V451 are promising; however, it is impossible to predict the level of success the candidate will have in late-stage clinical trials.
“CSL’s focus is to produce a safe and effective vaccine. It is important that on completion of clinical trials, the public has confidence in UQ-CSL V451, which makes use of the well-established recombinant protein technology platform and Seqirus’s proprietary adjuvant MF59, which has an extensive safety track record in humans,” Perreault said.
UQ is currently undertaking a phase 1 clinical study on UQ-CSL V451 to assess safety and immunogenicity in healthy volunteers.
UQ Vice-Chancellor Professor Deborah Terry said, “We are proud of the contributions that UQ researchers have been making in response to the COVID-19 pandemic.
“UQ has a reputation for transitioning its innovations and discoveries to delivering solutions for the world’s challenges. Federal and Queensland governments, philanthropists and donors, a multitude of research collaborations and particularly our remarkable partnership with CEPI and CSL have made our accelerated timeline possible. And we are grateful to the Queenslanders who have stepped up for our phase 1 trials, with an overwhelming response to our recent call for volunteers aged 56 and over.”
If the phase 1 study proves to be successful towards the end of this year, CSL will take responsibility for the subsequent phase 2b/3 clinical trial, which is expected to commence in late 2020.
The Oxford/Astra Zeneca agreement
Completing a comprehensive assessment of its capabilities, CSL has agreed with AstraZeneca to manufacture approximately 30 million doses of the AZD1222 vaccine candidate, with first doses planned for release to Australia early next year. AZD1222 requires a two dose per person regime. CSL will manufacture the vaccine from its Australian facilities and schedule production around the UQ-CSL V451 manufacture, as well as manufacture of the company’s vital core therapies.
Expanding Australia’s COVID-19 vaccine manufacturing readiness
The Australian Government will provide funding to support CSL’s readiness to manufacture AZD1222, thus expanding Australia’s on-shore COVID-19 vaccine manufacturing capabilities. This funding will be used to establish at-risk components required to produce the commercial manufacture of a recombinant vector-based COVID-19 vaccine, including the acquisition of specialised equipment, recruitment, training and redeployment of personnel and retooling and reconfiguration of existing manufacturing facilities to current Good Manufacturing Practice standards.
AstraZeneca has established a separate commercial arrangement with the Australian Government to supply the AZD1222 vaccine to Australia once it successfully completes clinical trials.
Successful manufacture and supply of AZD1222 under contract to AstraZeneca is subject to Australian regulatory approval.
“We are pleased that we can produce the AZD11222 without compromising the production of our core products — influenza vaccines and plasma and recombinant protein therapies — and provide a second option for a COVID-19 vaccine candidate to Australia,” Perreault said.
“Acknowledging that CSL is the only company in Australia with manufacturing facilities capable of producing this vaccine, we thank the Australian Government for their support, ensuring Australia has access to onshore COVID-19 vaccine production and supply.
“Our facilities will require modifications in order to fulfil the compliance requirements for working with vector-based vaccines, as well as the addition of skilled personnel and further capital investment.
“While there are still a number of milestones to be met, we are hopeful that by next year we’ll be in the fortunate position of having a vaccine candidate to support Australia and the world’s emergence from this crisis.
“CSL’s history is linked with a commitment to Australia’s public health needs. In 1918 we produced a vaccine for the Spanish influenza pandemic, in 2009 we developed a vaccine for the swine flu pandemic and now we are incredibly proud to use our skills, technology and facilities to help ensure Australia — and the world — can access a COVID-19 vaccine,” Perreault stated.
Further reading
Recombinant vaccines
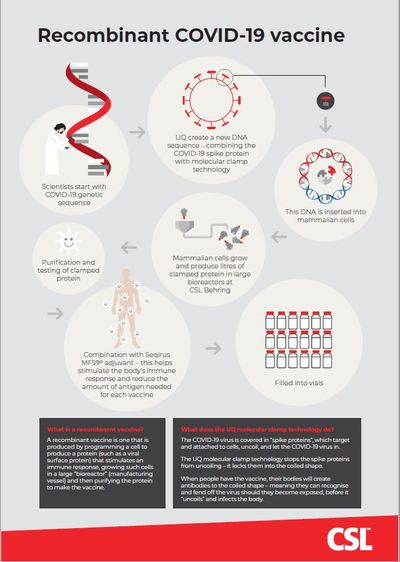
A recombinant vaccine is one that is produced by programming a cell to produce a protein (such as a viral surface protein) that stimulates an immune response, growing such cells in a large ‘bioreactor’ (manufacturing vessel) and then purifying the protein to make the vaccine. This is called antigen. Recombinant vaccines use a well-established and stable platform, already in use in other vaccines such as the HPV vaccine (Gardasil) and the Hepatitis B vaccine.
For a clearer image, click here.
UQ molecular clamp technology
The COVID-19 virus is covered in ‘spike proteins’, which target and attach to cells, uncoil and let the COVID-19 virus in. The UQ molecular clamp technology stops the spike proteins from uncoiling — it locks them into the coiled shape.
When people have the vaccine, their bodies will create antibodies to the coiled shape — meaning they can recognise and fend off the virus should they become exposed, before it ‘uncoils’ and infects the body.
MF59 adjuvant
Adjuvants are added to vaccines to improve immune response and to reduce the amount of antigen needed for each vaccine, enabling more doses to be manufactured more rapidly. Influenza vaccines containing Seqirus’s proprietary adjuvant MF59 have been widely tested in clinical trials and used in clinical practice for more than 20 years. Over 150 million doses have been administered over this time — including in Seqirus’s H1N1 pandemic vaccine.
CSL Behring Broadmeadows Biotech Manufacturing Facility (BMF)
Opened in 2014, the Biotechnology Manufacturing Facility (BMF) is a world-class facility and one of the largest and most advanced of its kind in the Southern Hemisphere. The facility uses biotechnology processes to grow genetically engineered mammalian cell-lines that express (make) specific proteins that can be collected and purified. Recombinant products potentially offer significant advantages for the treatment of a range of rare and serious disease, including bleeding disorders, inflammatory conditions and cancer, and the BMF plays a key role in CSL’s research and global drug development strategy.
Medicine shortages are testing Australia's health system and clinicians need national support
Medicine shortages are no longer isolated supply disruptions. They are escalating and directly...
Mindfulness and endoscopies — a more effective combination?
A UK study involving 231 patients has advocated 'mindful endoscopy' — allowing...
How do different types of pain influence empathy?
Different types of pain influence how unpleasantly we perceive it but also how we empathise with...




![[New Zealand] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89856/wfmedia_thumb/..jpg)
![[Australia] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89855/wfmedia_thumb/..jpg)



