Patients co-design invasive heart surgery monitoring clinical trial
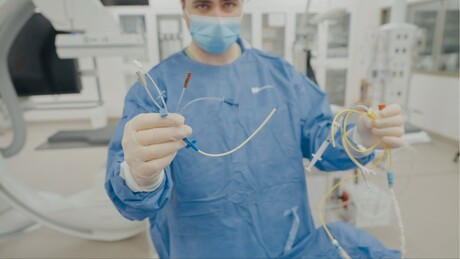
Patients and their families have co-designed a clinical trial to determine if invasive devices used to monitor cardiac function during and after surgery help or harm the more than 20,000 Australians who go under the knife for open heart surgery each year.
Led by the Monash Victorian Heart Institute, the 2000-patient PUMA trial will compare the commonly used pulmonary artery catheter (PAC) and the central venous catheter (CVC); the latter being a less invasive, simpler alternative that, researchers hope, will reduce unnecessary treatments, shorten ICU stays, reduce healthcare costs, and significantly cut the environmental footprint of intensive care by 2030.
“These invasive devices have been broadly de-adopted in other high-risk patient groups after large clinical trials due to sepsis, acute respiratory distress syndrome, and use in non-cardiac surgery failing to show a benefit to patients,” said lead investigator Dr Luke Perry, Head of Anaesthetic Research at the Victorian Heart Institute. “PUMA will resolve decades of international controversy around the role of pulmonary artery catheters in contemporary practice, paving the way for high-value care that could reduce major complications and get patients home to their families sooner.”

The insights of lived-experience investigator Paige Druce, a two-time cardiac surgery survivor, shaped the study’s approach and priorities from the earliest pilot phase. “It is important to feel confident that every procedure is truly necessary,” Druce said. “Co-designing clinical trials with people who have lived experience is important because it helps to make sure the research focuses on things that matter to the people it’s meant to help.” Druce added: “It also ensures that the research is done in a way that feels fair and respectful to those taking part.”
Associate Professor Lachlan Miles, PUMA co-investigator and Head of Research in the Department of Anaesthesia at Austin Health, said the study will gather important information to ensure the best patient outcomes. “Pulmonary artery catheters provide important information about heart function,” Miles said. “Unfortunately, they are not without risk, and could even trigger unnecessary treatments, increase complications and prolong hospital admissions.”
Endorsed by the Australia and New Zealand College of Anaesthetists Clinical Trials Network, recruitment will begin in Australia and internationally this year.
Phone: 03 9905 4000
Pplus Medical Digital Storage Device (DSD) for platelets
Pplus Medical's Digital Storage Device (DSD) provides platelets with optimal storage and...
SorbaView SHIELD integrated securement dressing
The SorbaView SHIELD multi-layer dressing and catheter securement system is designed to provide a...
LipidPlan in a box ASCVD resources
LipidPlan in a box is intended to support GPs and primary care teams in undertaking QI activities...

![[New Zealand] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89856/wfmedia_thumb/..jpg)
![[Australia] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89855/wfmedia_thumb/..jpg)




