Antimicrobial sutures can help reduce SSI risk
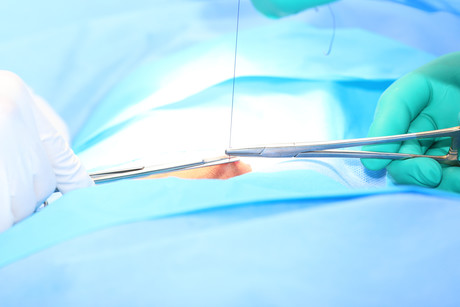
Ethicon, part of Johnson & Johnson Medical Devices, has welcomed the release of the updated Australian Guidelines for the Prevention and Control of Infection in Healthcare, in particular its suggestion that using antimicrobial-coated sutures can help to reduce the risk of surgical site infections (SSIs).
The Guidelines, produced by the National Health and Medical Research Council’s (NHMRC) in partnership with the Australian Commission on Safety and Quality in Health Care (the Commission), provide recommendations that outline the critical aspects of infection prevention and control.
The Guidelines state that “Using antimicrobial-coated sutures (included on the ARTG e.g. triclosan-coated sutures) can help to reduce SSI rates” in the hospital setting. Antimicrobial sutures, such as those coated in triclosan, can help to reduce a patient’s risk of acquiring an infection resulting from surgery by up to 28%.2
SSI are among the most common healthcare-associated infections (HAI) in Australia; they increase morbidity and mortality in surgical patients and represent an economic burden to the healthcare system.1 In fact, Australian data shows that the average cost of an SSI may be associated with $23,097 in extra costs.3,4
There are multiple reasons why an SSI can occur; however, the risk is increased when a foreign body is introduced, such as sutures.5 Like all foreign bodies, when implanted, sutures are rapidly coated with tissue proteins, creating sites for bacterial colonisation.5 This colonisation can lead to biofilm formation, which can also increase the difficulty of treating an infection.5
Numerous peer reviewed, randomised clinical trials, as well as prospectively planned meta-analyses of these trials, support a growing body of evidence that antibacterial sutures are an important tool in the fight against surgical site infections.2
The Guidelines’ reference to antimicrobial-coated sutures adds to the growing support from other reputable organisations, including the World Health Organization, the Centers for Disease Control, the American College of Surgeons and the Surgical Infection Society, on the positive impact triclosan-coated sutures have on reducing the risk for SSI.6,7,8
Ethicon believes their Ethicon Plus Sutures are the only commercially available sutures in Australia coated with triclosan that inhibit bacteria that are commonly associated with SSIs (including S. aureus, S. epidermidis, MRSA, MRSE, E. coli and K. pneumoniae) from colonizing the suture9,10,11.
References:
- Australian Commission on Safety and Quality in Healthcare (2017). Approaches to Surgical Site Infection Surveillance. Available online at https://www.safetyandquality.gov.au/wp-content/uploads/2017/07/Approaches-to-Surgical-Site-Infection-Surveillance.pdf. Accessed on 1st May 2018
- de Jonge SW, Atema JJ, Solomkin JS, Boermeester MA. Meta-analysis and trial sequential analysis of triclosan-coated sutures for the prevention of surgical site infection. Brit J Surg. 2017;ePub-DOI: 10.1002/bjs.10445.
- Australian Institute of Health and Welfare. Admitted patient care 2015-16: Australian Hospital Statistics. Accessed 30/05/2019 at https://www.aihw.gov.au/getmedia/3e1d7d7e-26d9-44fb-8549-aa30ccff100a/20742.pdf.aspx?inline=true
- Independent Hospital Pricing Authority (AU).National Hospital Cost Data Collection 2015-16, acute admitted episodes, excluding same day. 5. Magill, S.S., et al., "Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida". Infection Control Hospital Epidemiology, 33(3):(2012): 283-91. Accessed April 6, 2016 at http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf.
- Edmiston CE, Daoud FC, Leaper D. Is there an evidence-based argument for embracing an antimicrobial (triclosan)-coated suture technology to reduce the risk for surgical-site infections?: A meta-analysis. Surgery 2013;154:89100.
- Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. doi:10.1001/jamasurg.2017.0904.
- Global guidelines on the prevention of surgical site infection. World Health Organization website. http://www.who.int/gpsc/ssi-prevention-guidelines/en/. Accessed April 4, 2017.
- Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg. 2016;224:59 74.
- Ming X, Rothenburger S, Nichols MM. In vivo and in vitro antibacterial efficacy of PDS Plus (polidioxanone with triclosan) suture. Surg Infect. 2008;9(4):451-457.
- Ming X, Rothenburger S, Yang D. In vitro antibacterial efficacy of Monocryl Plus Antibacterial Suture (poligelcaprone 25 with triclosan). Surg Infect. 2007;8(2):201-207.
- Rothenburger S, Spangler D, Bhende S, Burkley D. In vitro antimicrobial evaluation of coated Vicryl Plus Antibacterial Suture (coated polyglactin 910 with triclosan) using zone of inhibition assays. Surg Infect. 2002;3(suppl):79-87.
$1bn vaccine and antivenom manufacturing facility opens
A $1 billion cell-based influenza vaccine and antivenom manufacturing facility has opened in...
National concussion clinical guidelines now available
The first Australia- and New Zealand-specific guidelines for all forms of concussion — from...
Doctors criticise "risky prescribing agenda"
The AMA and RACGP have expressed disappointment in the Pharmacy Board of Australia's...



![[New Zealand] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89856/wfmedia_thumb/..jpg)
![[Australia] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89855/wfmedia_thumb/..jpg)




