Improving success rates: lactic acid in IVF
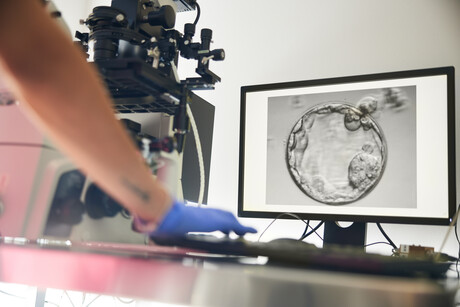
The co-author of research published this year in Biomolecules explains how the metabolism of the embryo could be a major component determining the success of implantation in IVF.
The process of embryo implantation, a key process in the establishment of a successful pregnancy, is complex. With approximately one in six couples in Australia facing infertility, many turn to IVF as a pathway to parenthood. While IVF success rates have significantly improved over the last 45 years — since the birth of the first test-tube baby — and around 5% of Australian children are born via IVF, more than half of couples attempting IVF are unable to establish a pregnancy. Our recent research1 suggests that the metabolism of the embryo could be a major component determining the success of implantation.
Invasion of the uterus by the embryo
Dr Kathryn Gurner — a clinical research embryologist and my co-author on this research — and I have been investigating how the unique metabolic functions of the late-stage preimplantation embryo (known as the blastocyst) can control events key in the uterus to promote implantation.
Implantation is a curious biological phenomenon as it represents the invasion of the mother’s uterus by the blastocyst stage embryo, an entity which is not considered as ‘self’ by the woman but rather is recognised as a foreign body. This raises fundamental questions like “how does the blastocyst prevent immune rejection by the woman” and “how does it then go on to create its own blood supply?” — a phenomenon referred to as angiogenesis.

From biochemistry to the blastocyst
While many researchers around the world have considered the process of implantation from the perspective of uterine signalling, Gurner and I have instead considered the potential role of the blastocyst’s unique metabolism; that being its capacity to form lactic acid even when given ample oxygen to oxidise it, in this intricate process.
This unusual metabolic characteristic is referred to as aerobic glycolysis and is a metabolic trait also seen in invasive tumours. By examining the mechanisms employed by cancer cells, we were able to explore whether the blastocyst utilises similar processes for implantation — with findings suggesting that this indeed seems to be the case.
Lactic acid — more than a by-product of the gym
Often associated with the burn of an intense workout, lactic acid is widely regarded as a by-product of anaerobic metabolism, occurring when oxygen is in short supply — such as in muscles during a sprint or heavy exercise.
However, in the case of the embryo, it transpires that lactic acid produced by the blastocyst is used to deliberately create a specific microenvironment around the embryo characterised by low pH and high levels of lactate. Essentially, the blastocyst forms a small acidic cloud around itself, which facilitates a number of crucial events in the early stages of implantation.
The initiation of implantation by lactic acid
In the initial phases, this acidic environment helps transition the uterus into a receptive state for implantation, a process known as endometrial receptivity. By influencing the function and expression of key genes within endometrial cells, lactic acid promotes changes that allow the uterine lining to become more hospitable for the attaching embryo.
Lactic acid also facilitates the blastocyst’s invasion of the uterus by driving alterations in gene and protein expression that encourage its penetration into the endometrial tissue. This invasion is crucial for embedding the embryo deeply within the uterine lining, ensuring proper nutrient exchange and sustained development.
Additionally, as endothelial cells — progenitors of blood vessels — and various immune cells within the uterine environment absorb lactate produced from the blastocyst, their function/behaviours adapt to promote implantation. In endothelial cells, this leads to an increase in the formation of new blood vessels, ensuring the embryo secures a consistent oxygen and nutrient supply.
Meanwhile, lactate uptake by immune cells influences the maternal immune response by dampening immune activator cells and increasing molecules that suppress immune activity. This adjustment prevents the embryo from being recognised as a foreign entity, facilitating successful implantation.
In conclusion
These discoveries highlight the intricate role of lactic acid in shaping the uterine environment during the earliest phases of pregnancy. While this research provides exciting insights, there is still much to uncover about lactate signalling in the uterus — and consequently this will help create ways in which we can pretreat patients to enhance uterine receptivity.
1. Gurner KH, Gardner DK. Blastocyst-derived lactate as a key facilitator of implantation. Biomolecules. 2025;15(1):100. doi: 10.3390/biom15010100

Patients co-design invasive heart surgery monitoring clinical trial
Patients and their families have co-designed a clinical trial to determine if invasive devices...
Have Australian researchers discovered the secret to treating sepsis?
Promising results from a Phase 2 clinical trial have Australian researchers looking to progress...
Could this tailored heart pump transform care for half of heart failure patients?
Half of the 64 million people living with heart failure have no access to heart pump treatments....

![[New Zealand] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89856/wfmedia_thumb/..jpg)
![[Australia] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89855/wfmedia_thumb/..jpg)




