The stent tech-race against heart attack

When it comes to the tiny scaffold-like stents inserted to unblock the clogged arteries of heart disease patients, the stakes couldn’t be higher.
The chances of a coronary stent failing are about 10 per cent, but if they fail the consequence of a sudden blockage can be catastrophic. For example while the risk of a blood clot forming on a stent is only 0.6 per cent per year, if it occurs the risk of death from a sudden heart attack is a sobering 40 per cent.
These potentially deadly consequences of failure are driving a global technological race to improve stents using everything from dissolving materials, embedded drugs, personalised 3D-printed devices, and even nanotechnology that could deliver treatments like anti-cholesterol drugs direct to arteries.
“We are dealing here with an uncommon complication, but it is a dreaded complication, so we need to be doing all we can to continually improve on stent technology,” says Professor Peter Barlis, cardiologist and medical researcher based at St Vincent’s Hospital Melbourne and the University of Melbourne. He stands on the frontline of this technological race.
“Even with all the advances that have been made in stent technology, we have found that there are still complications arising from arteries re-narrowing despite the application of a stent, and those complications can be abrupt, leading to sudden and potentially fatal heart attacks.”
Professor Barlis is leading research teams on stent technology that are rapidly blurring the lines between medicine, physics and engineering. When he takes time out to speak with Pursuit he is fresh from performing an operation at St Vincent’s to insert into a heart patient the latest in approved stent technology, a dissolving device made from magnesium metal. It is the first time the stent has been used in Victoria and is only one of four such stents inserted across the country.
Developed by German medical device company BIOTRONIKS, the Magmaris magnesium stent was only approved for clinical use in Europe in June 2016.
The attraction of dissolvable stents over permanent stents is that there is less risk of the body eventually reacting against the device and causing inflammation and scar tissue that can block the artery again. By the time the stent dissolves the artery should be fully healed and stay open. And being made from metal, it offers greater strength and reliability than current dissolvable stents that are made from polymer or plastic.
While Professor Barlis wasn’t involved in developing Magmaris, he had worked on the feasibility of magnesium stents a decade ago during his time at Imperial College London. He now sees his ongoing research into engineering better stents as a crucial part of his own clinical practice.
INCREASED STRENGTH
“They call me the plumber because I go and unblock arteries, and it is enormously rewarding to be involved in life or death situations when someone has had a massive heart attack and I have to open up the arteries again. But modern day clinicians can’t just be working in hospitals seeing patients. Clinicians have to be out there innovating to improve patient outcomes,” he says.
“The magnesium stent is attractive because it affords us increased strength over the polymer stents and dissolves in just 12-18 months rather than three years or more. But we still need to improve these devices and find further innovations.”
The central challenge for stent technology is to make them as thin as possible while also being strong. Stents need to be thin and flexible so that when inserted they can safely navigate the complex network of arteries around the heart that are just 3-4 millimetres wide. But they also have to be strong enough to do the job of opening up the artery and clearing plaque build up. Our arteries can turn back on themselves by more than 90 degrees, and the pattern of arteries is so unique from person to person that Professor Barlis likens the network around the heart to fingerprints.
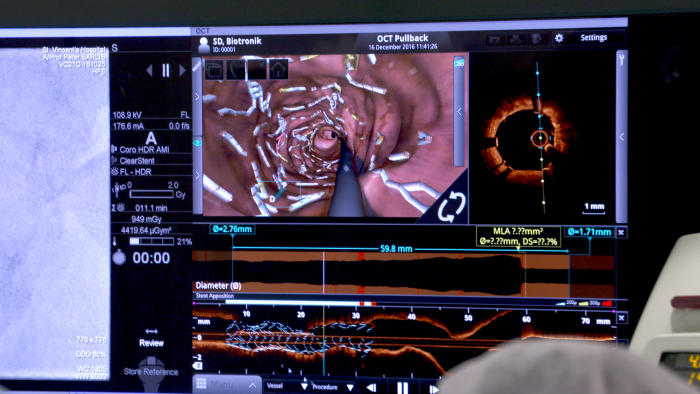
“The challenge we face in biomedical engineering is creating devices that give flexibility but also afford us the strength to keep the artery open.”
In the 1980s stents were made from bare stainless steel or similar alloys, but in almost half of all cases there would be an eventual complication such as inflammation and scarring. The big advance came in 2003 with the development of drug-eluting stents. These are stents that are coated with a polymer that binds with anti-inflammatory or immune-suppressing drugs to stop the body reacting against the device. Professor Barlis says these drug-eluting stents reduced the risk of arteries re-narrowing to 10 per cent.
The next big advance was the development of full polymer stents that could dissolve and leave just carbon dioxide and water that is readily reabsorbed by the body. But to match the strength of metal stents, the polymer stents have to be thicker at about 150 microns, which is about three times the width of a human hair. That makes them less flexible than metal stents that can be 60-90 microns.
Professor Barlis also points out there are now concerns that polymer stents may be taking too long to dissolve, putting patients at greater risk of complications.
A three-year study published in the Lancet indicates that a particular dissolvable polymer stent was taking longer than expected to dissolve and that the consequent risk of arteries re-narrowing was higher than in drug-eluting metal stents.
The magnesium stent is coated with a polymer that binds to an immune suppressant drug, Sirolimus. Like the polymer stents, the magnesium stent dissolves and is reabsorbed as an inert substance, just like the magnesium nutrients that already exist in our bodies. However, because magnesium is weaker than other metal stents, it has to be as thick as the polymer stents, which Professor Barlis says may prevent it from being used easily in areas where there are acute bends in the arteries.
USING NANOTECHNOLOGY
“Our vision is to improve these devices by making them thinner, which may mean looking at other materials. But there are also opportunities for potentially using nanotechnology to use stents as platforms to release drugs over time to where they are most needed – in the artery itself. We may be able to lock drugs into tiny nano-sized particles and control their release.
“We could potentially use such an advance to target cholesterol in the artery to stop the build-up of the plaque that eventually causes blockages. It could also be a way of delivering drugs such as blood thinners, for example, without having to rely on patients to keep taking their medication.”
Professor Barlis is also investigating the potential to create custom-built stents using 3D printing and designed to fit in the unique bends of an individual’s arteries. At the moment the 3D printing resolution isn’t high enough but he believes it is just a matter of time.
“All the stents we are using come in a set size, but our arteries are not a set size. We are all very different. So customising stents to fit may be another way of reducing the risk of complications.
“These are the sorts of areas where my mind is going when I contemplate the future of stents because what we need most of all in this area is innovation. And that means bringing together mechanical and chemical engineering with pharmacology and 3D printing.
“We have to be thinking ahead to how we prevent patients turning up on our operating tables in the first place.”
Clearly this is one plumber who wouldn’t mind going out of business. “That would do me,” he laughs. “A place in research would be just fine.”
This article was first published on Pursuit. Read the original article.
Originally published here.
GenesisCare expands with $35m Northern Beaches cancer centre
The relocated centre has expanded its services with a new radiation therapy offering and access...
In Conversation with Royal Women's Hospital CEO Sue Matthews
An hour after the final call for visitors to leave, Professor Sue Matthews — now CEO of...
Global prostate cancer rates predicted to double by 2040
The number of annual prostate cancer deaths worldwide is predicted to rise by 85% from 375,000 in...










